In the search for environmental sustainability, the textile sector faces a major challenge: wastewater treatment. Light-activated polymers have emerged as a possible approach for wastewater decontamination, demonstrating excellent efficacy.
In an article published in the journal Chemosphere, researchers in Brazil describe the synthesis of several different semiconductor materials that can be used as photoanodes.
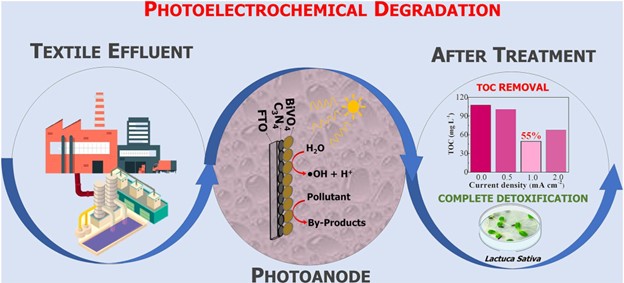
The Researcher team created semiconductors using carbon nitride (C3N4) and bismuth vanadate (BiVO4). Bismuth vanadate, which is related with the utilization of solar energy, effectively removes harmful chemicals while using less electricity. The findings indicate that the material, which is inexpensive and simple to manufacture, has potential applications in industry, particularly for treating wastewater from textile mills. Production of the material can be integrated with the generation of hydrogen, which can then be used to generate energy.
The researchers are affiliated with the Center for Development of Functional Materials (CDMF) and the Center for Innovation in New Energies (CINE).
Key Highlights from the research:
- The C3N4–BiVO4 mixture showed better photoelectrocatalytic properties.
- The photocurrent of the C3N4–BiVO4 is 1.5 mA cm−2
- The charge mobility increases 10 times in the C3N4–BiVO4 mixture.
- PhEC detoxification and 55 % TOC in 300 min using C3N4–BiVO4 photoanode
Why do they choose Semiconductor as photoanodes rather than photocatalysts?:
Semiconductors are inexpensive and abundant in nature, their application as photoanodes has great potential. Photocatalytic efficiency is determined by the catalyst’s performance, which must include a small band gap for a wide range of light absorption, separation, efficient charge transport, and strong redox capabilities. It is almost impossible to combine all of these qualities in a single catalyst. To overcome these technological application restrictions, a variety of materials have been developed, including semiconductor-semiconductor composites, catalysts, doping, and carbon-based coupling.
Bismuth Vanadate (BiVO4) & Carbon Nitride (C3N4) as photoanodes:
Bismuth vanadate (BiVO4) has strong chemical stability, outstanding photochemical characteristics, and a broad range of solar excitation. It has great potential for use in photoelectrochemistry. Pure BiVO4 has disadvantages such as low electrical conductivity, slow surface kinetics, and electron/hole pair recombination. Carbon nitride (C3N4) has received substantial research because it is a metal-free semiconductor that is ecologically friendly, simple to make, and has intriguing photocatalytic capabilities.
Findings indicate that the C3N4-BiVO4 heterostructure enhances the separation of photogenerated carriers while providing promising photoelectrochemical performance. Few researchers have assessed this form of electrode and demonstrated its potential, none have dedicated themselves to employing it for the degradation of genuine effluents or detoxification, indicating a significant gap in the literature that must be addressed.
A C3N4-BiVO4 heterostructured photoelectrocatalyst was created utilizing a simple and quick method for removing organic matter and detoxifying genuine textile industry effluent. The photoelectrocatalytic characteristics of the synthesized materials (C3N4, BiVO4, BiVO4-C3N4, and C3N4-BiVO4) were assessed using a solar simulator. After the effluent degradation process, the toxicity of the reaction solutions was initially assessed using a model based on Lactuca sativa.
Findings and effective outcomes from the work in perspective to industries:
- C3N4–BiVO4 heterostructure exhibited the highest photoelectrocatalytic charge transfer constant, mobility, and lifetime of charge carriers
- The photoelectric degradation of the effluent demonstrated substantial removal of Total Organic Carbon (TOC) and the generation of low toxicity degradation products, accompanied by low energy consumption
- The compelling results underscore the high potential of the synthesized C3N4–BiVO4 heterostructure for industrial applications, particularly in addressing environmental challenges associated with textile industry effluents.